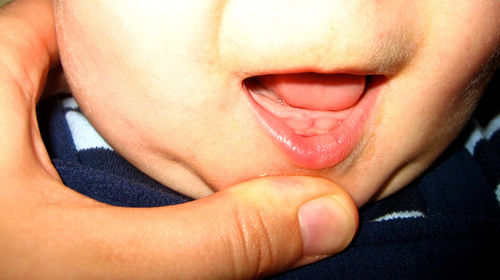
The U.S. Food and Drug Administration warned yesterday against using a lidocaine solution as a pain reliever on teething babies’ gums, saying it can cause deaths and serious injuries in infants and toddlers.
Viscous lidocaine is a prescription medication, a local anesthetic in a gel-like syrup. Reports of overdoses in teething babies have been linked to jitteriness, confusion, vision problems, vomiting, falling asleep too easily, shaking and seizures.
FDA has previously recommended that parents and caregivers not use benzocaine products for children younger than 2 years, except under the advice and supervision of a health care professional. Benzocaine, which, like viscous lidocaine, is a local anesthetic, can be found in such OTC products as Anbesol, Hurricaine, Orajel, Baby Orajel, and Orabase.
The use of benzocaine gels and liquids for mouth and gum pain can lead to a rare but serious—and sometimes fatal—condition called methemoglobinemia, a disorder in which the amount of oxygen carried through the blood stream is greatly reduced. And children under 2 years old appear to be at particular risk.
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day